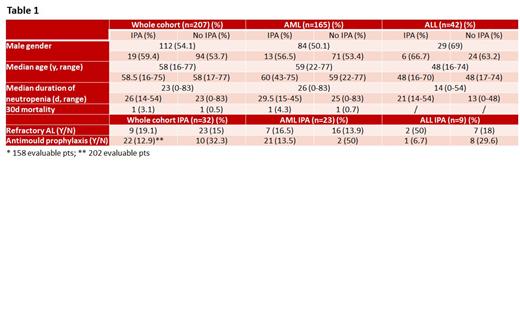
Introduction. Invasive pulmonary aspergillosis (IPA) still affects the outcome of acute leukemia (AL) patients (pts). Although this aspect is better defined in acute myeloid leukemia (AML) ( Candoni et al, Mycoses 2020), some reports show a dismal impact also in acute lymphoid leukemia (ALL) ( Cattaneo et al, Leuk Lymphoma 2019). However, data concerning the epidemiology of IPA in adult ALL patients, as well as the best antifungal prophylaxis strategy, are still lacking. Therefore, to describe the incidence of IPA in AL pts and their clinical characteristics, as well as the predisposing factors, we carried out a prospective multicentric observational study within the Rete Ematologica Lombarda (REL). Methods. All consecutive AL patients diagnosed during a 3-year period and treated with curative intent were recorded. Diagnosis of IPA was defined according to EORTC/MSG criteria ( Donnelly et al, Clin Infect Dis 2020). Data concerning age, gender, AL type, chemotherapy (cht) received, complete remission (CR) achievement, antifungal prophylaxis, duration of neutropenia and 30-day mortality were collected. Patient's characteristics were analysed by standard descriptive statistics. The Student's t-test was used to compare continuous values; the Fisher's exact test was used to compare differences in percentage. P values below 0.05 were considered statistically significant. Results. Between 2018 and 2020, 207 AL pts (AML: 165, ALL: 42) were evaluated in five Hospitals participating to the REL. Median age of the whole cohort was 58y, and M/F ratio 112/95. After induction treatment, CR was achieved in 153 of the 200 evaluable pts (76.5%), more frequently in ALL (38/42, 90.5%) than in AML pts (115/158, 72.8%) (p=0.0146). During induction cht, proven/probable (p/p) and possible (poss) IPA were diagnosed in 32/207 pts (15.4%), equally divided in p/p and poss (16 each, 7.7%). P/p IPA were more frequent in ALL than in AML (ALL: 7/42, 16.6% vs AML: 9/165, 5.4%; p= 0.0235); similarly, considering also poss IPA, incidence was higher, even if not significantly (9/42, 21.4% vs 23/165, 13.9%; p=0.2374). Among ALL pts, IPA was more frequent in pts not achieving CR (2/4, 50%) than CR pts (7/38, 18%), whereas in AML CR achievement did not influence IPA frequency (CR group: 15/115, 13%; no CR group: 7/43: 16.5%). ALL pts were uniformly treated with pediatric-like protocols; in AML pts no statistically significant differences in IPA incidence were observed according to cht received (3+7/like: 13/81, 16%; 3+7+midostaurin: 2/27, 7.4%; FLAG-Ida: 3/21, 14.3%; CPX-351: 4/19, 21%). Antimould prophylaxis was protective against IPA in 202 evaluable pts (Yes: 22/171, 12.9%; No: 10/31, 32.3%, p=0.0134). Among ALL pts only 1 out of 15 (6.7%) receiving antimould prophylaxis developed IPA, as compared to 8/27 (29.6%) of those not receiving antimould prophylaxis (p=0.1235). All but two AML pts developing IPA were on posaconazole prophylaxis. Both mean age and duration of neutropenia were significantly lower in ALL than in AML pts (46.02y vs 57.75y, p<0.0001 and 16.66d vs27.96d, p<0.0001); however, an impact of mean duration of neutropenia on IPA incidence was observed only in ALL pts (IPA group: 24.38d; no IPA group: 14.79d, p=0.0359). Only 2 AML pts died at 30d: 1 (3.1%) out of 32 pts with IPA and 1 (0.6%) out of 175 pts without. No early death was observed in ALL pts. Table 1 summarizes the clinical characteristics of AL pts in relation to IPA. Conclusions. Despite younger age, shorter duration of neutropenia and higher percentage of CR achievement, IPA, particularly p/p, was more frequently observed in ALL pts than AML during induction cht. High dose induction cht seems to have no impact in IPA incidence in AML pts. On the other hand, intensive cht ALL protocols, including high doses of dexamethasone, could have a role in IPA development, as well as the lack of antimould prophylaxis, which is confirmed as a predictive factor for IPA. Therefore, studies with antimould agents as prophylaxis against aspergillosis in ALL pts during inductions would be desirable.
Disclosures
Cattaneo:Pfizer, Jazz: Other: travel grants. Fracchiolla:Abbvie, Jazz, Pfizer, Amgen: Speakers Bureau; Abbvie, Jazz, Pfizer, Amgen: Other: travel grants. Borlenghi:Amgen, Incyte: Other: travel grants; AbbVie, BMS: Consultancy. Rossi:Abbvie: Honoraria. Tucci:Janssen: Other; Takeda: Other; Gentili: Other; Sanofi: Other; Eli Lilly: Other; Kiowa Kiryn: Other; Beigene: Other. Lussana:Amgen: Speakers Bureau; Clinigen: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Membership on an entity's Board of Directors or advisory committees; Incyte: Speakers Bureau; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal